#> Warning: This tutorial was written with Giotto version 0.3.6.9048, your version
#> is 1.0.3.This is a more recent version and results should be reproduciblelibrary(Giotto) # 1. set working directory results_folder = '/path/to/directory/' # 2. set giotto python path # set python path to your preferred python version path # set python path to NULL if you want to automatically install (only the 1st time) and use the giotto miniconda environment python_path = NULL if(is.null(python_path)) { installGiottoEnvironment() }
Dataset explanation
The CODEX data to run this tutorial can be found here Alternatively you can use the getSpatialDataset to automatically download this dataset like we do in this example.
Goltsev et al. created a multiplexed datasets of normal and lupus (MRL/lpr) murine spleens using CODEX technique. The dataset consists of 30 protein markers from 734,101 single cells. In this tutorial, 83,787 cells from sample “BALBc-3” were selected for the analysis.
Dataset download
# download data to working directory # use method = 'wget' if wget is available. This should be much faster. getSpatialDataset(dataset = 'codex_spleen', directory = results_folder, method = 'wget')
Part 1: Giotto global instructions and preparations
# 1. (optional) set Giotto instructions instrs = createGiottoInstructions(show_plot = FALSE, save_plot = TRUE, save_dir = results_folder, python_path = python_path) # 2. create giotto object from provided paths #### expr_path = paste0(results_folder, "codex_BALBc_3_expression.txt.gz") loc_path = paste0(results_folder, "codex_BALBc_3_coord.txt") meta_path = paste0(results_folder, "codex_BALBc_3_annotation.txt")
Part 2: Create Giotto object & process data
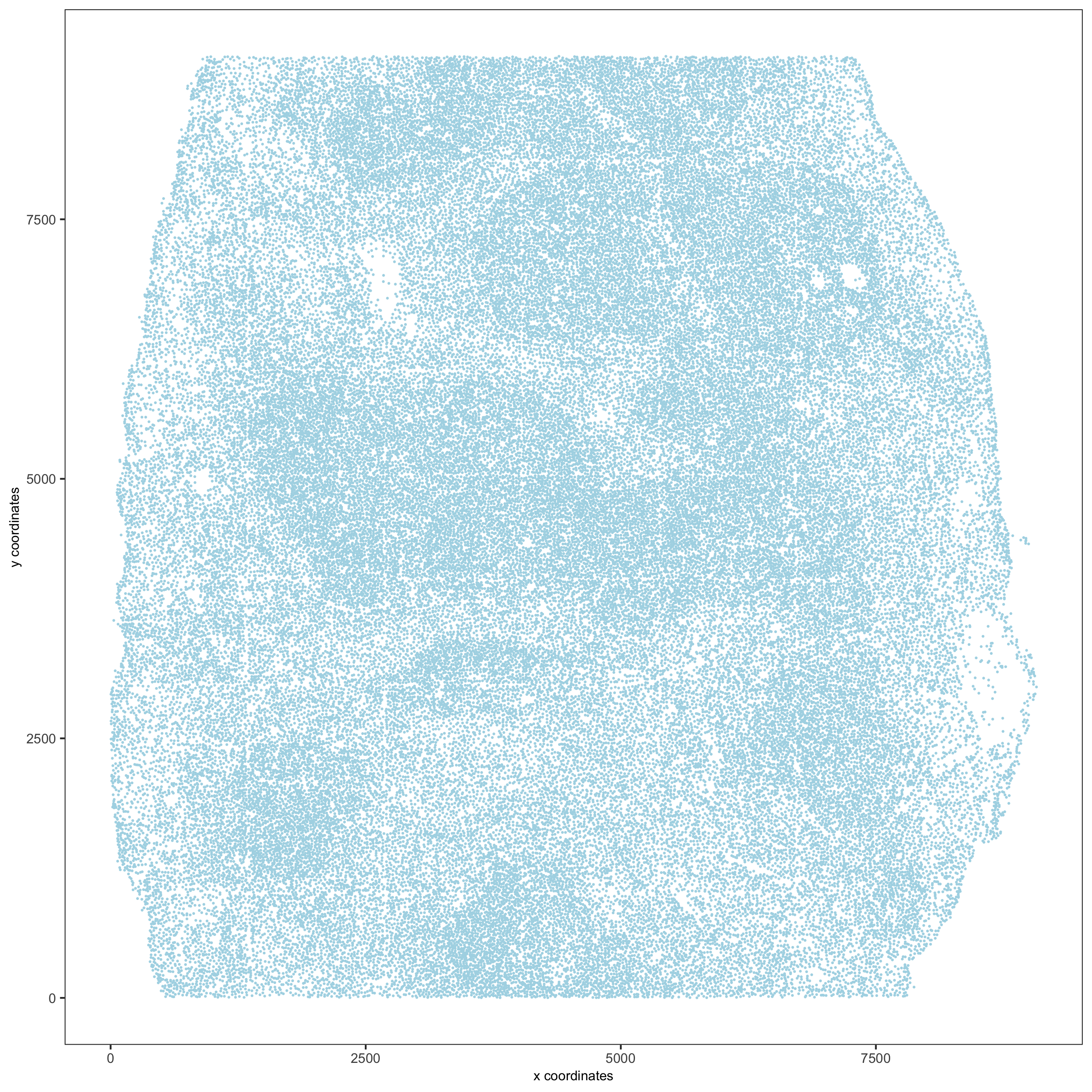
# read in data information # expression info codex_expression = readExprMatrix(expr_path, transpose = F) # cell coordinate info codex_locations = data.table::fread(loc_path) # metadata codex_metadata = data.table::fread(meta_path) ## stitch x.y tile coordinates to global coordinates xtilespan = 1344; ytilespan = 1008; # TODO: expand the documentation and input format of stitchTileCoordinates. Probably not enough information for new users. stitch_file = stitchTileCoordinates(location_file = codex_metadata, Xtilespan = xtilespan, Ytilespan = ytilespan); codex_locations = stitch_file[,.(Xcoord, Ycoord)] # create Giotto object codex_test <- createGiottoObject(raw_exprs = codex_expression, spatial_locs = codex_locations, instructions = instrs, cell_metadata = codex_metadata) # subset Giotto object cell_meta = pDataDT(codex_test) cell_IDs_to_keep = cell_meta[Imaging_phenotype_cell_type != "dirt" & Imaging_phenotype_cell_type != "noid" & Imaging_phenotype_cell_type != "capsule",]$cell_ID codex_test = subsetGiotto(codex_test, cell_ids = cell_IDs_to_keep) ## filter codex_test <- filterGiotto(gobject = codex_test, expression_threshold = 1, gene_det_in_min_cells = 10, min_det_genes_per_cell = 2, expression_values = c('raw'), verbose = T) codex_test <- normalizeGiotto(gobject = codex_test, scalefactor = 6000, verbose = T, log_norm = FALSE,library_size_norm = FALSE, scale_genes = FALSE, scale_cells = TRUE) ## add gene & cell statistics codex_test <- addStatistics(gobject = codex_test,expression_values = "normalized") ## adjust expression matrix for technical or known variables codex_test <- adjustGiottoMatrix(gobject = codex_test, expression_values = c('normalized'), batch_columns = NULL, covariate_columns = NULL, return_gobject = TRUE, update_slot = c('custom')) ## visualize spatPlot(gobject = codex_test,point_size = 0.1, coord_fix_ratio = NULL,point_shape = 'no_border', save_param = list(save_name = '2_a_spatPlot'))
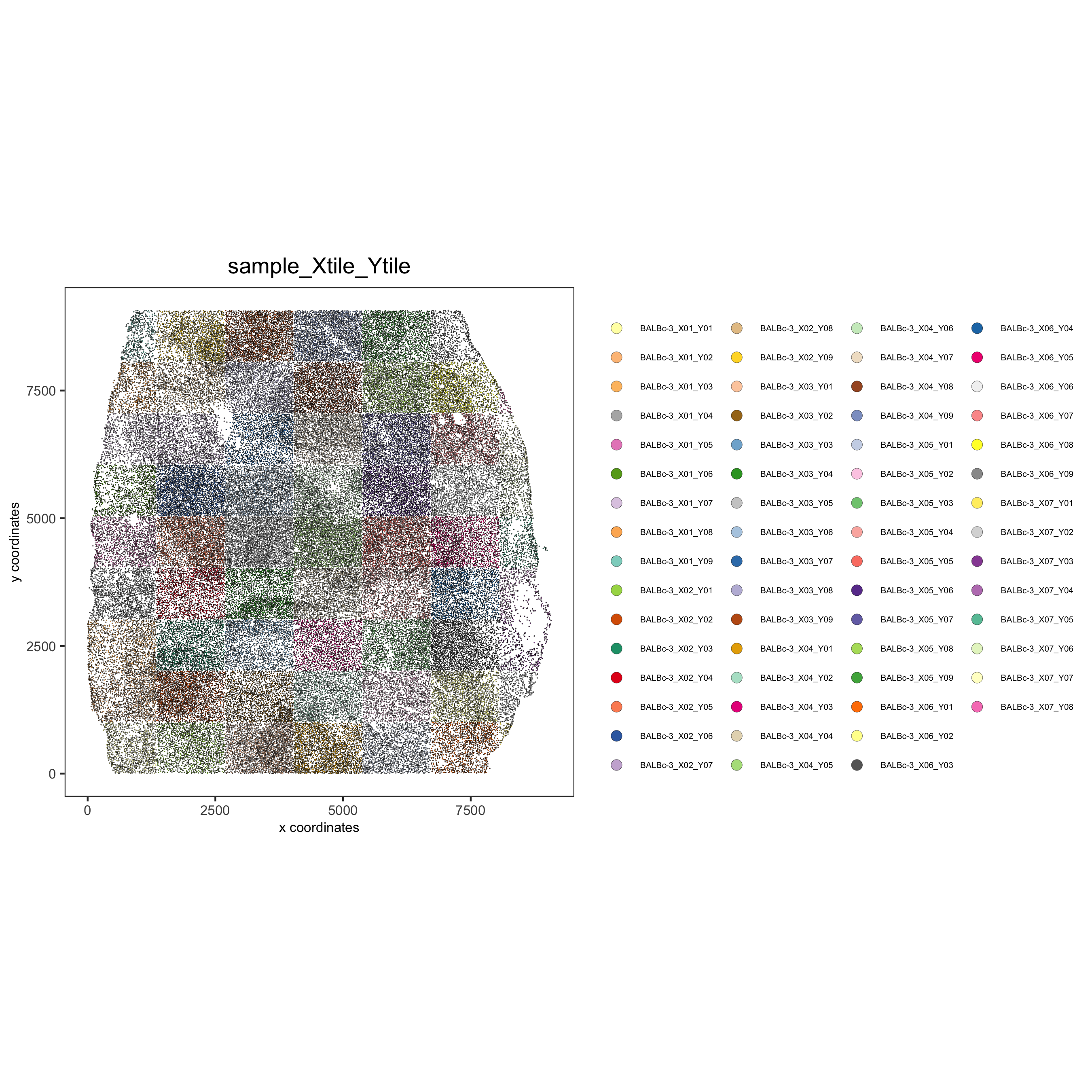
spatPlot(gobject = codex_test, point_size = 0.2, coord_fix_ratio = 1, cell_color = 'sample_Xtile_Ytile', legend_symbol_size = 3,legend_text = 5, save_param = list(save_name = '2_b_spatPlot'))
Part 3: Dimension reduction
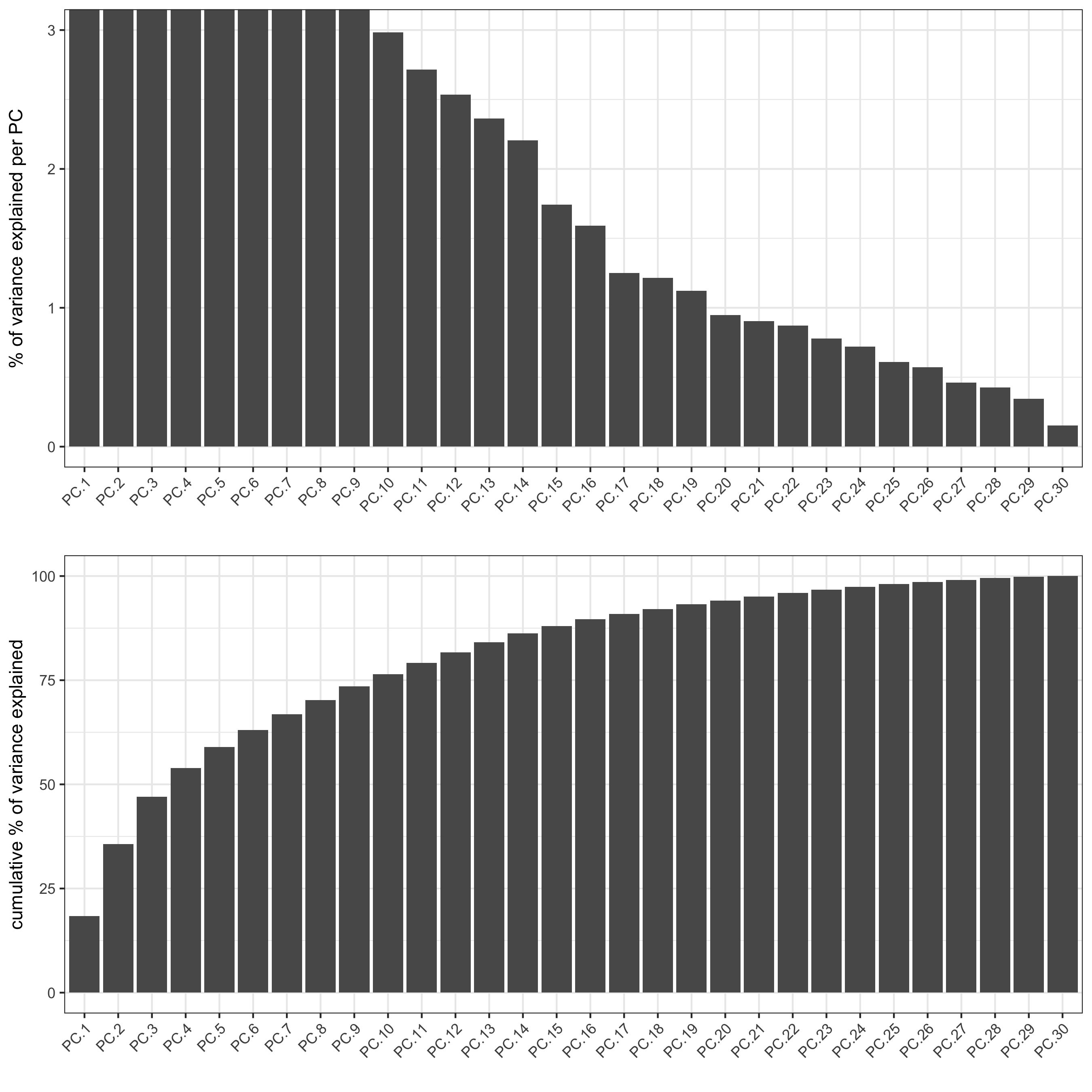
# use all Abs # PCA codex_test <- runPCA(gobject = codex_test, expression_values = 'normalized', scale_unit = T, method = "factominer") signPCA(codex_test, scale_unit = T, scree_ylim = c(0, 3), save_param = list(save_name = '3_a_spatPlot'))
plotPCA(gobject = codex_test, point_shape = 'no_border', point_size = 0.2, save_param = list(save_name = '3_b_PCA'))
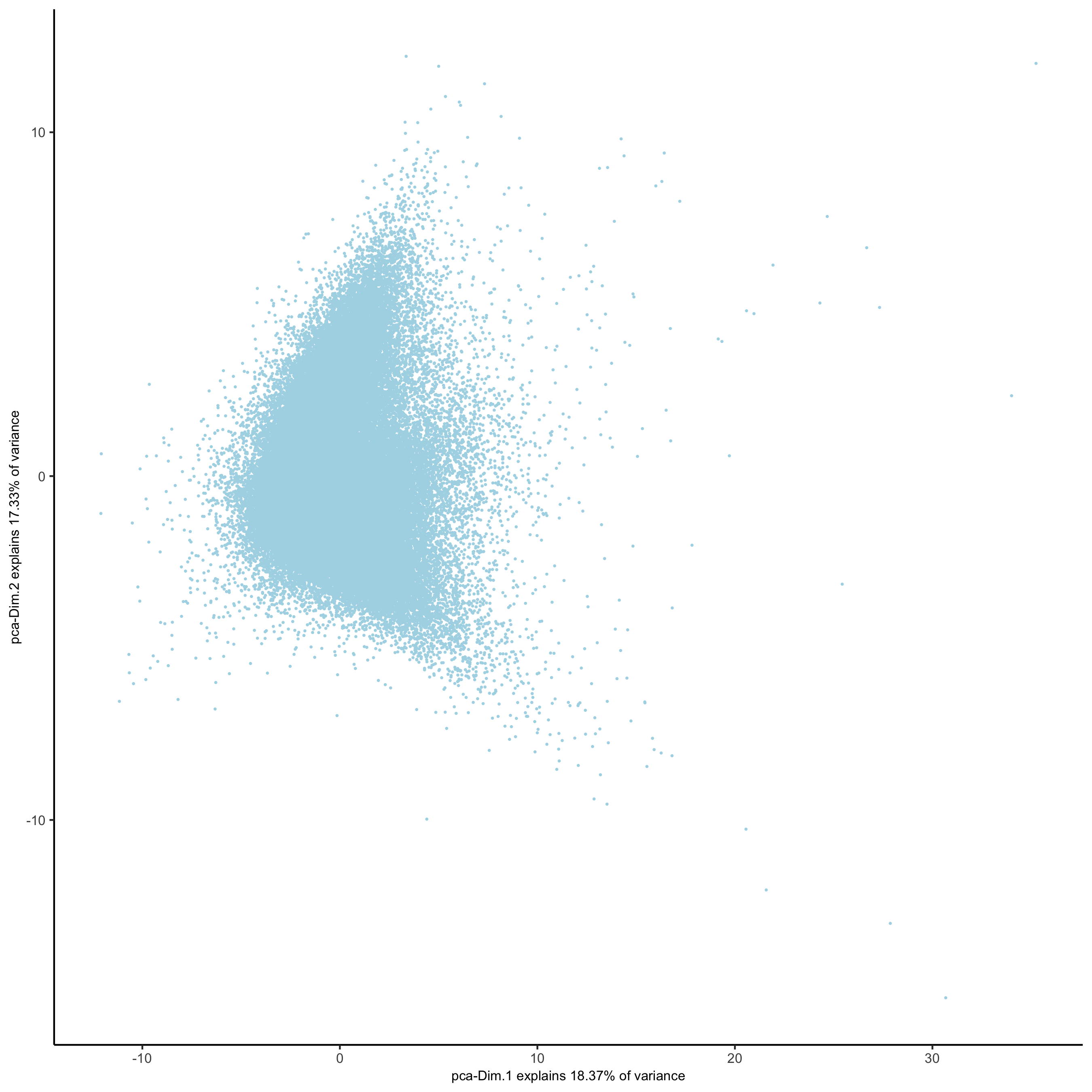
# UMAP codex_test <- runUMAP(codex_test, dimensions_to_use = 1:14, n_components = 2, n_threads = 12) plotUMAP(gobject = codex_test, point_shape = 'no_border', point_size = 0.2, save_param = list(save_name = '3_c_UMAP'))
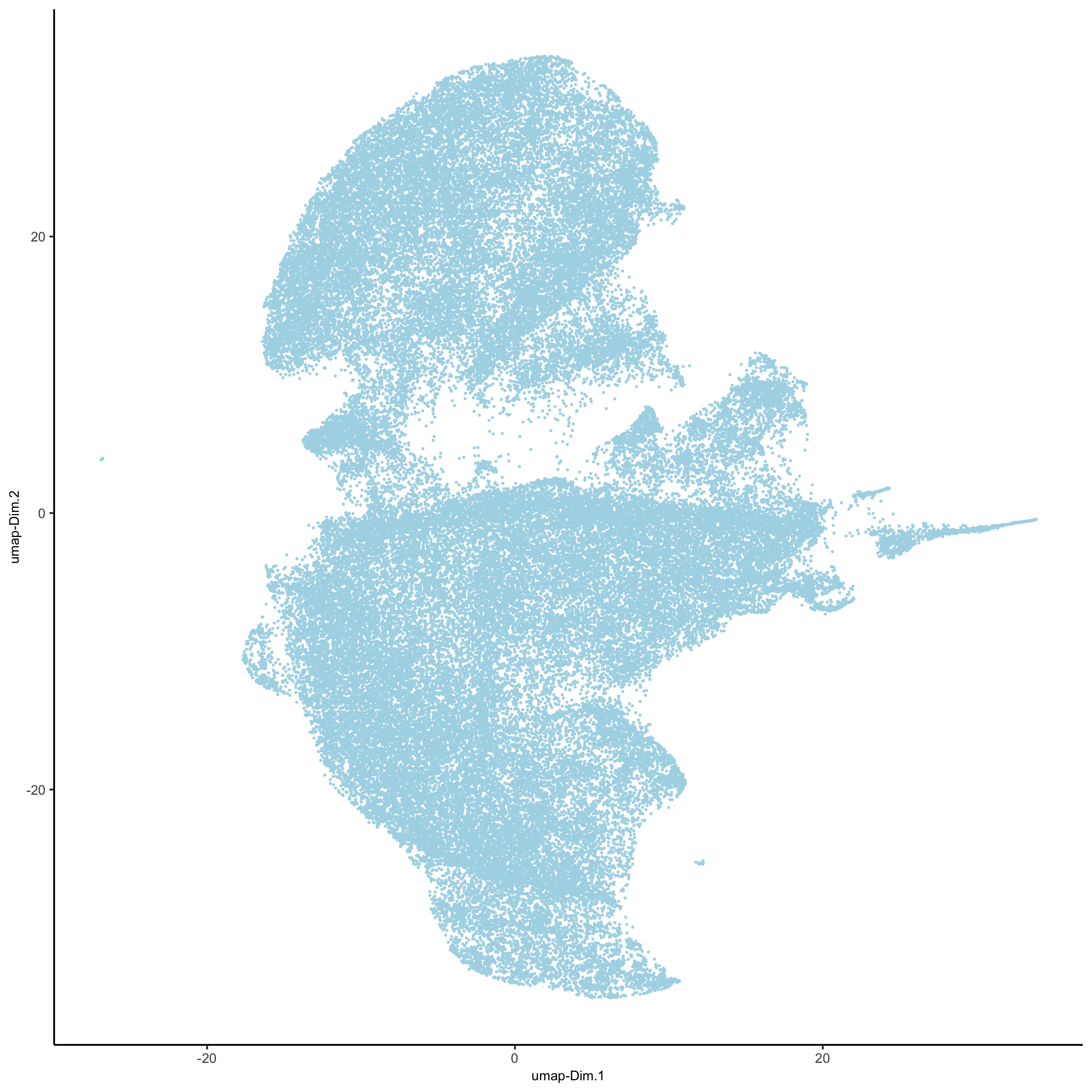
Part 4: Cluster
## sNN network (default) codex_test <- createNearestNetwork(gobject = codex_test, dimensions_to_use = 1:14, k = 20) ## 0.1 resolution codex_test <- doLeidenCluster(gobject = codex_test, resolution = 0.5, n_iterations = 100, name = 'leiden',python_path = python_path) codex_metadata = pDataDT(codex_test) leiden_colors = Giotto:::getDistinctColors(length(unique(codex_metadata$leiden))) names(leiden_colors) = unique(codex_metadata$leiden) plotUMAP(gobject = codex_test, cell_color = 'leiden', point_shape = 'no_border', point_size = 0.2, cell_color_code = leiden_colors, save_param = list(save_name = '4_a_UMAP'))
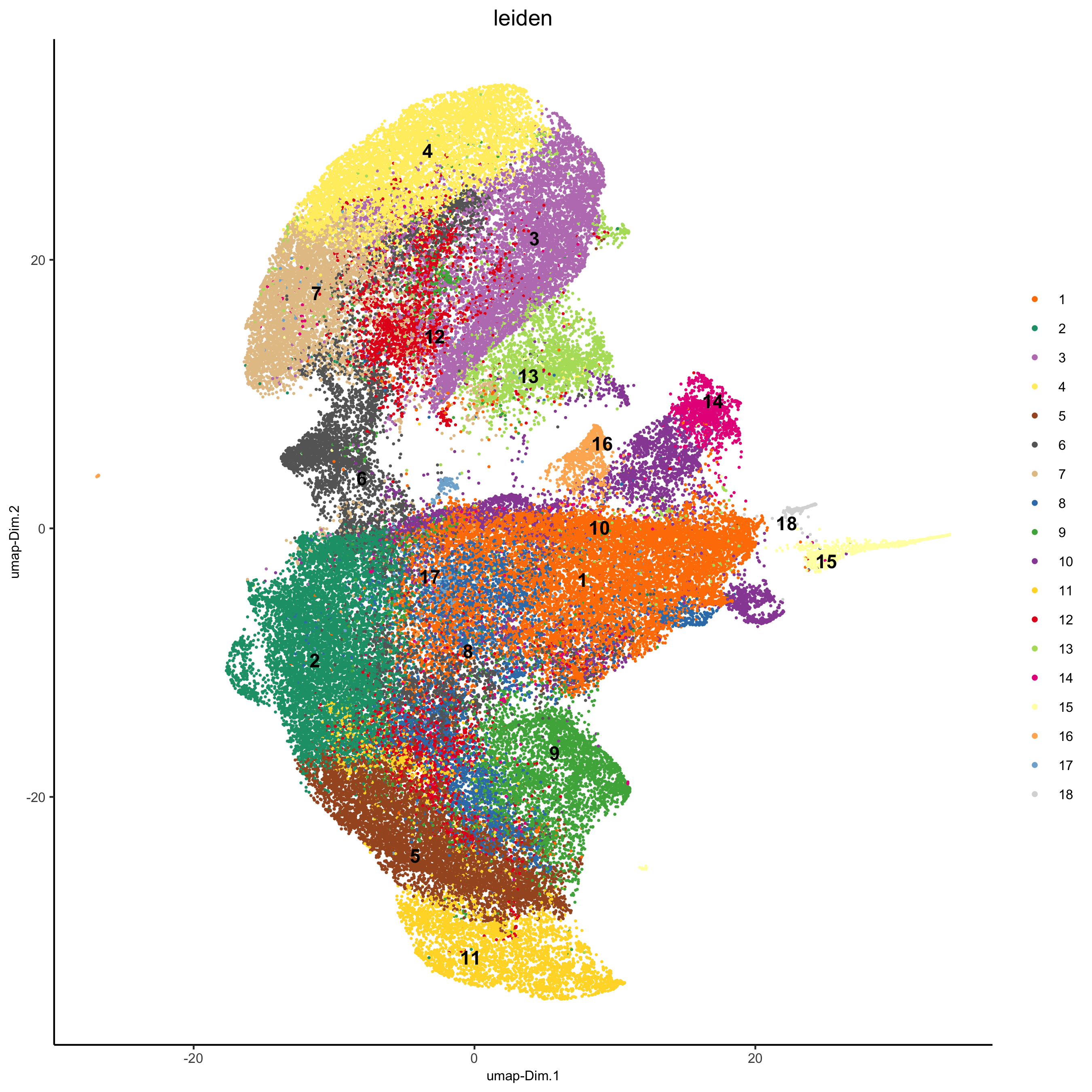
spatPlot(gobject = codex_test, cell_color = 'leiden', point_shape = 'no_border', point_size = 0.2, cell_color_code = leiden_colors, coord_fix_ratio = 1,label_size =2, legend_text = 5,legend_symbol_size = 2, save_param = list(save_name = '4_b_spatplot'))
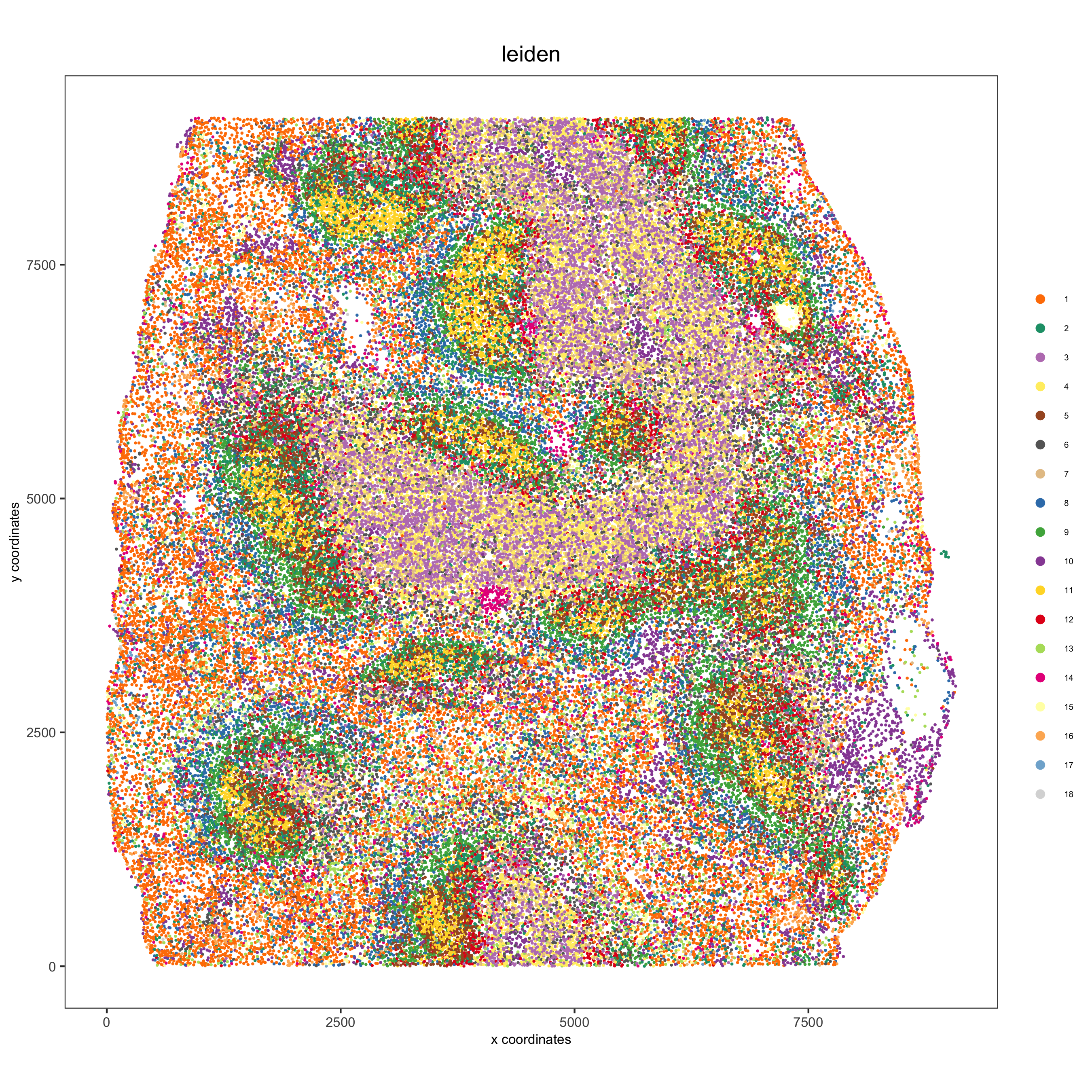
Part 5: Co-visualize
spatDimPlot2D(gobject = codex_test, cell_color = 'leiden', spat_point_shape = 'no_border', spat_point_size = 0.2, dim_point_shape = 'no_border', dim_point_size = 0.2, cell_color_code = leiden_colors,plot_alignment = c("horizontal"), save_param = list(save_name = '5_a_spatdimplot'))

Part 6: Differential expression
# resolution 0.5 cluster_column = 'leiden' markers_scran = findMarkers_one_vs_all(gobject=codex_test, method="scran", expression_values="norm", cluster_column=cluster_column, min_genes=3) markergenes_scran = unique(markers_scran[, head(.SD, 5), by="cluster"][["genes"]]) plotMetaDataHeatmap(codex_test, expression_values = "norm", metadata_cols = c(cluster_column), selected_genes = markergenes_scran, y_text_size = 8, show_values = 'zscores_rescaled', save_param = list(save_name = '6_a_metaheatmap'))
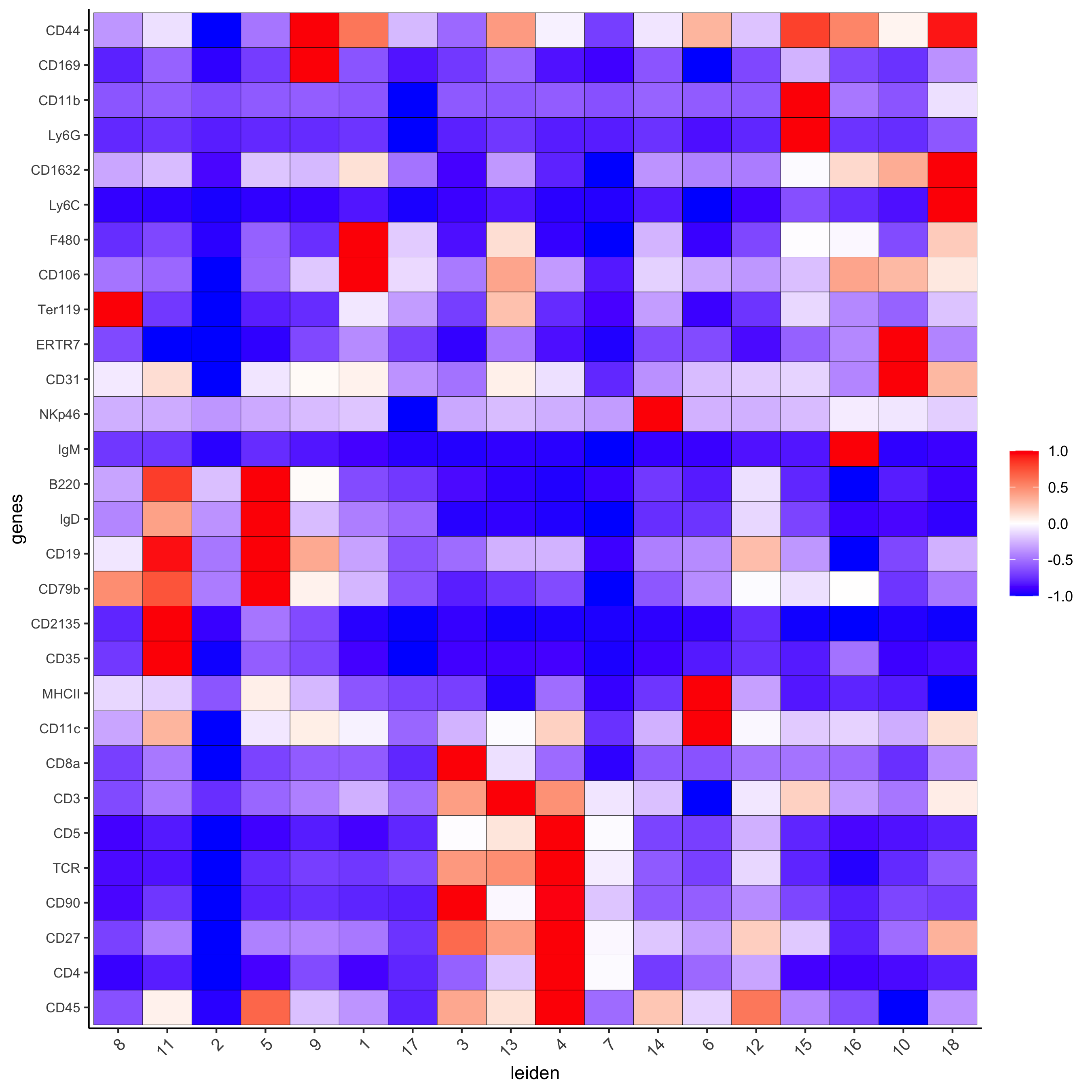
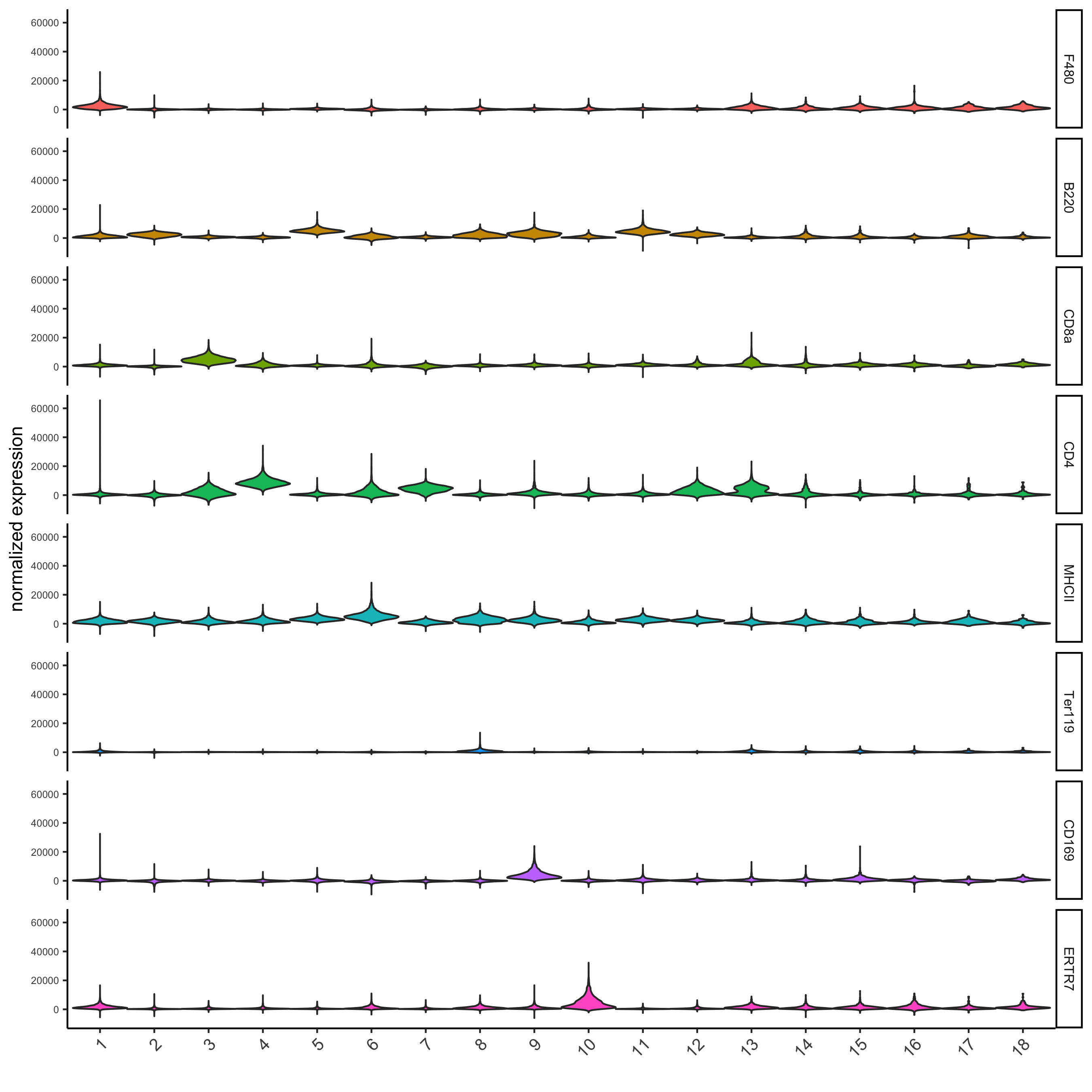
topgenes_scran = markers_scran[, head(.SD, 1), by = 'cluster']$genes violinPlot(codex_test, genes = unique(topgenes_scran)[1:8], cluster_column = cluster_column, strip_text = 8, strip_position = 'right', save_param = list(save_name = '6_b_violinplot'))
# gini markers_gini = findMarkers_one_vs_all(gobject=codex_test, method="gini", expression_values="norm", cluster_column=cluster_column, min_genes=5) markergenes_gini = unique(markers_gini[, head(.SD, 5), by="cluster"][["genes"]]) plotMetaDataHeatmap(codex_test, expression_values = "norm", metadata_cols = c(cluster_column), selected_genes = markergenes_gini, show_values = 'zscores_rescaled', save_param = list(save_name = '6_c_metaheatmap'))
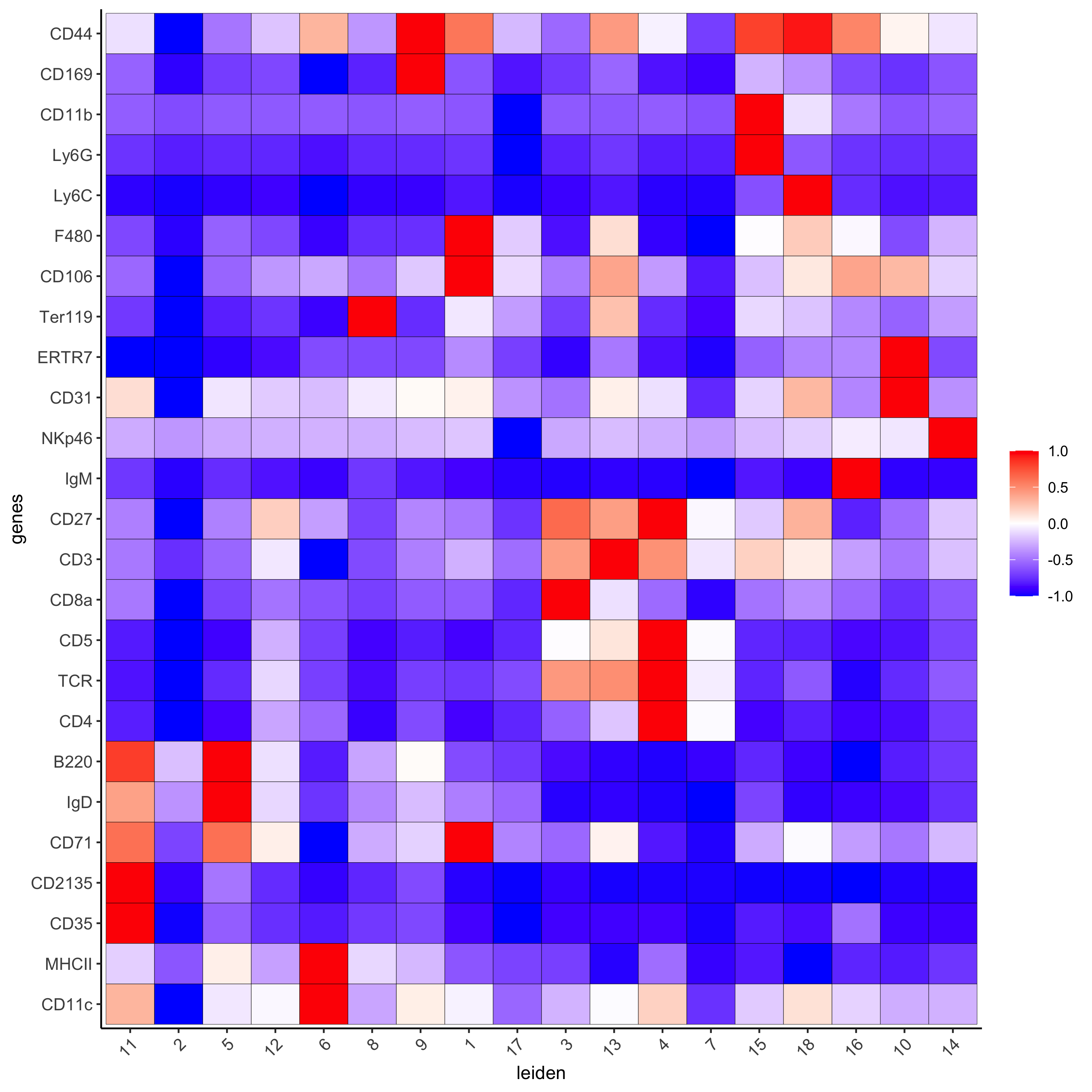
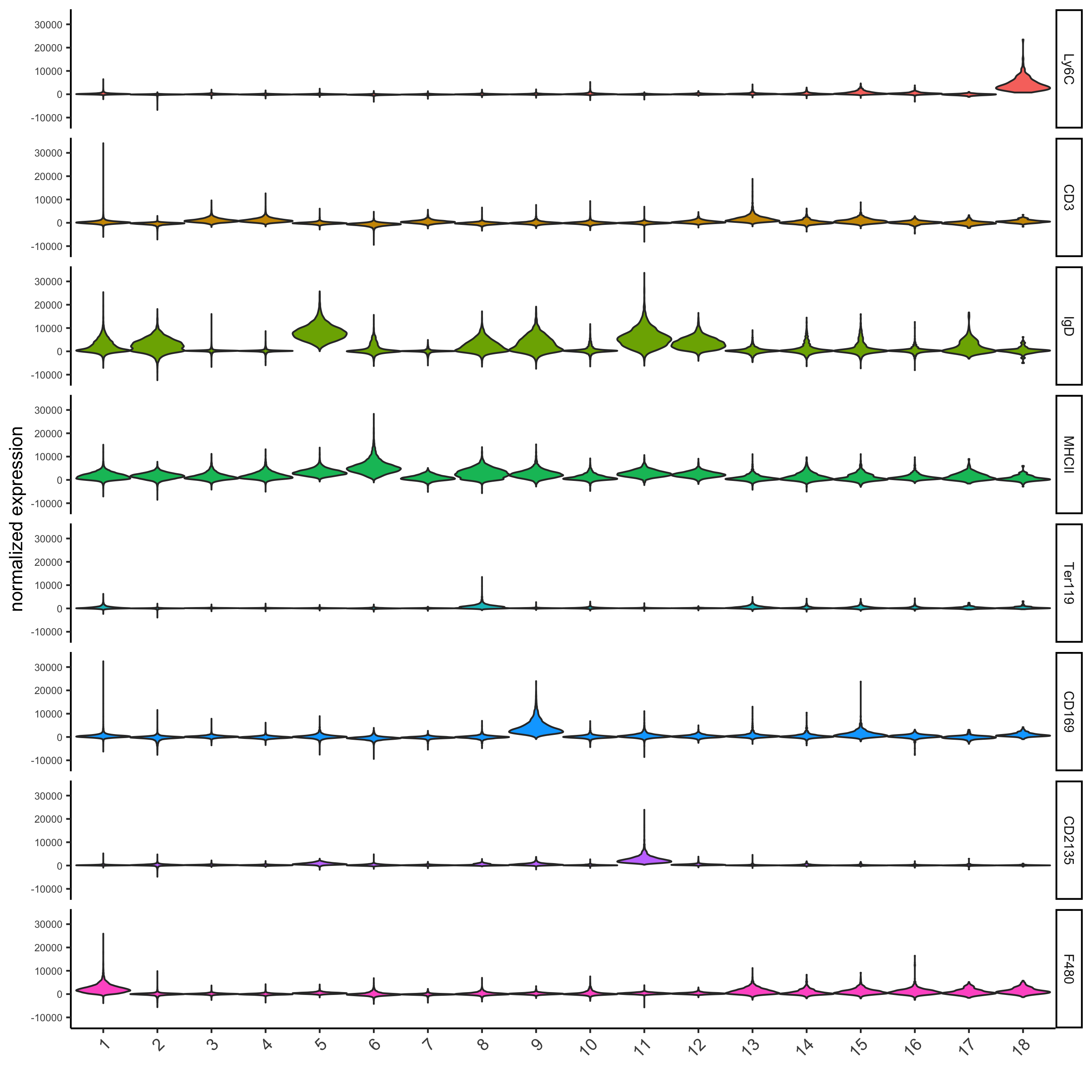
topgenes_gini = markers_gini[, head(.SD, 1), by = 'cluster']$genes violinPlot(codex_test, genes = unique(topgenes_gini), cluster_column = cluster_column, strip_text = 8, strip_position = 'right', save_param = list(save_name = '6_d_violinplot'))
Part 7: Cell type annotation
clusters_cell_types = c('erythroblasts-F4/80(+) mphs','B cells','CD8(+) T cells', 'CD4(+) T cells', 'B cells','CD11c(+)MHCII(+) cells', 'CD4(+) T cells','Ter119(+)', 'marginal zone mphs', 'CD31(+)ERTR7(+)', 'FDCs', 'B220(+) DN T cells', 'CD3(+) other markers','NK cells','granulocytes', 'plasma cells','ambiguous','CD44(+)CD1632(+)Ly6C(+) cells') names(clusters_cell_types) = c(1:18) codex_test = annotateGiotto(gobject = codex_test, annotation_vector = clusters_cell_types, cluster_column = 'leiden', name = 'cell_types') plotMetaDataHeatmap(codex_test, expression_values = 'scaled', metadata_cols = c('cell_types'),y_text_size = 6, save_param = list(save_name = '7_a_metaheatmap'))
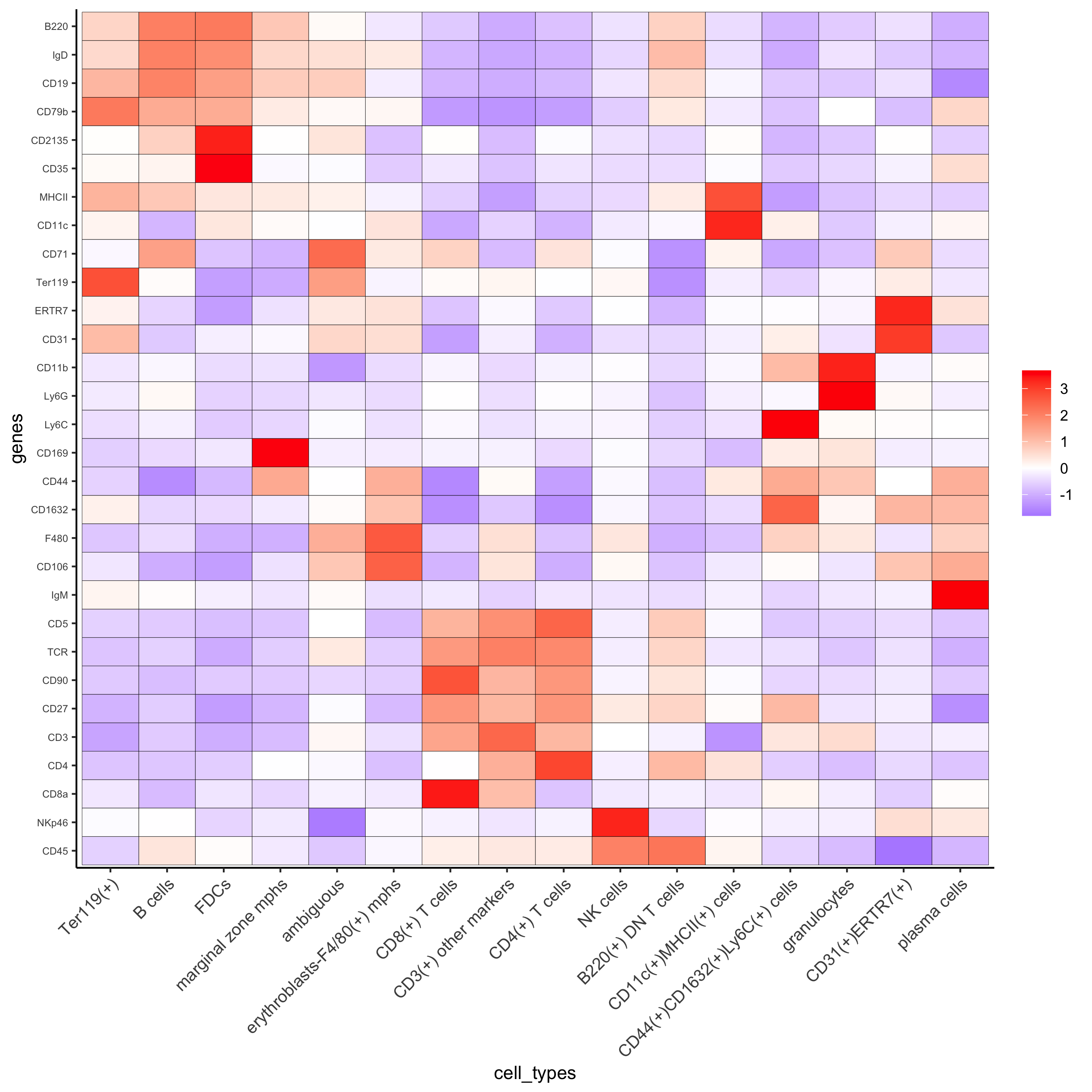
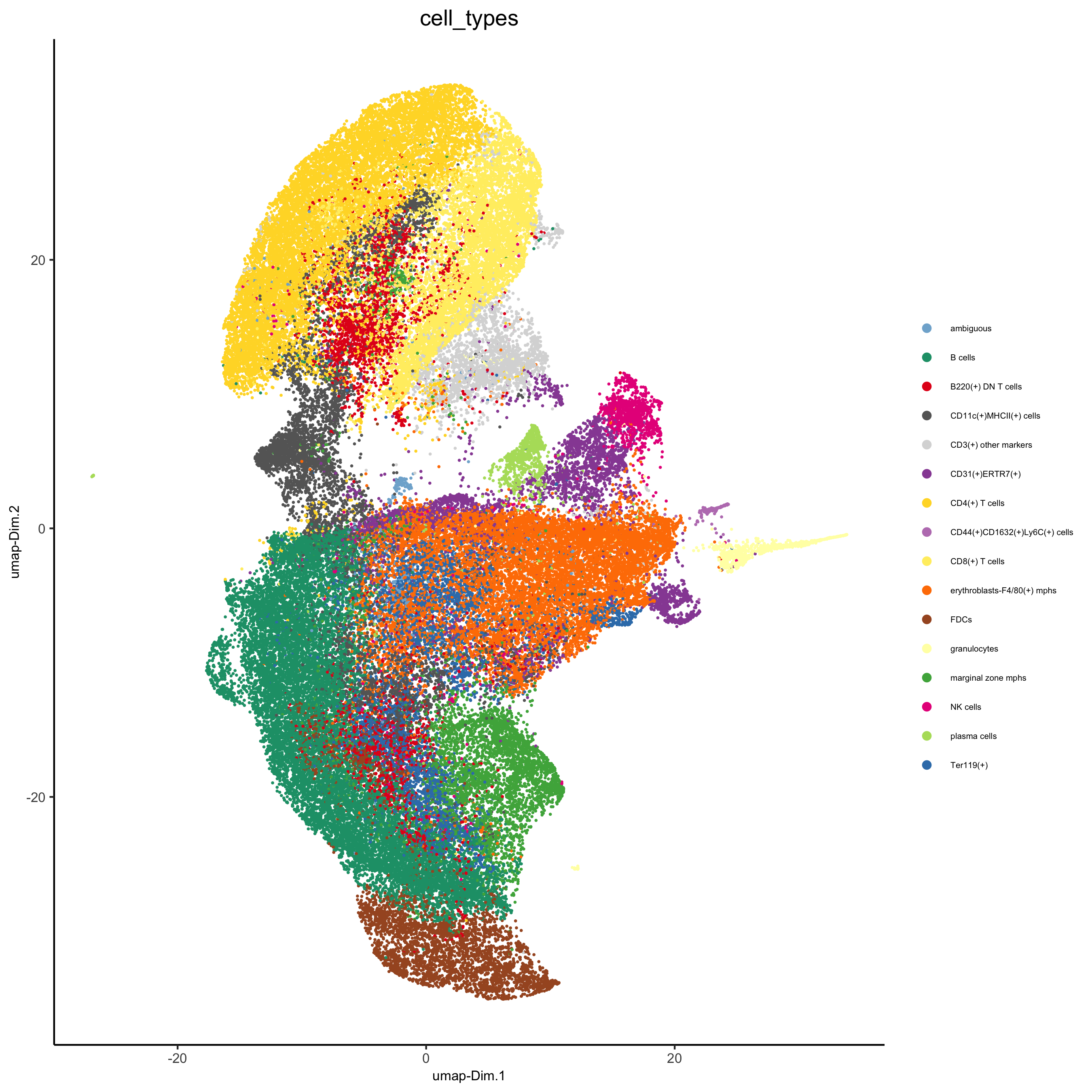
# create consistent color code mynames = unique(pDataDT(codex_test)$cell_types) mycolorcode = Giotto:::getDistinctColors(n = length(mynames)) names(mycolorcode) = mynames plotUMAP(gobject = codex_test, cell_color = 'cell_types',point_shape = 'no_border', point_size = 0.2, cell_color_code = mycolorcode, show_center_label = F, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '7_b_umap'))
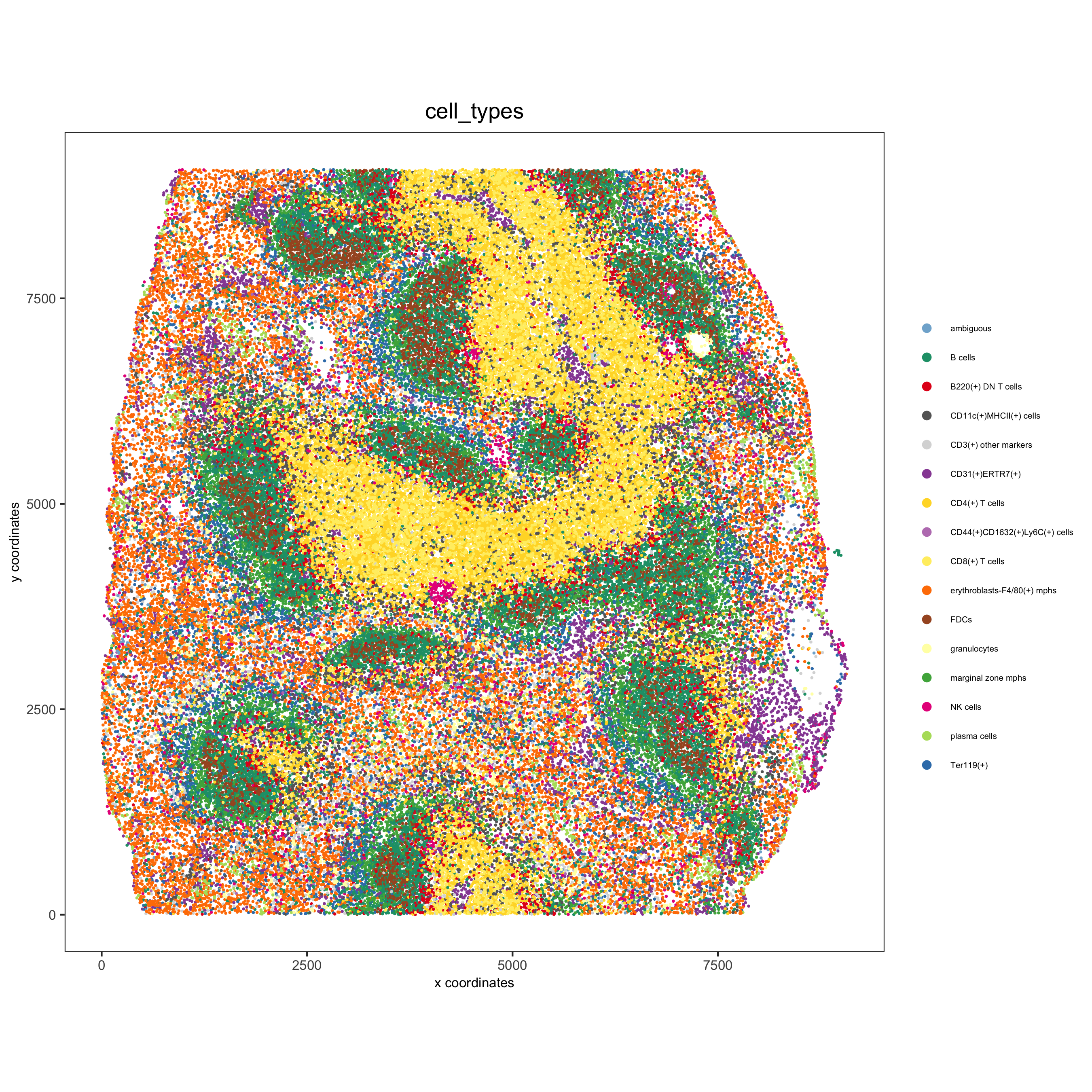
spatPlot(gobject = codex_test, cell_color = 'cell_types', point_shape = 'no_border', point_size = 0.2, cell_color_code = mycolorcode, coord_fix_ratio = 1, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '7_c_spatplot'))
part 8: Visualize cell types and gene expression in selected zones
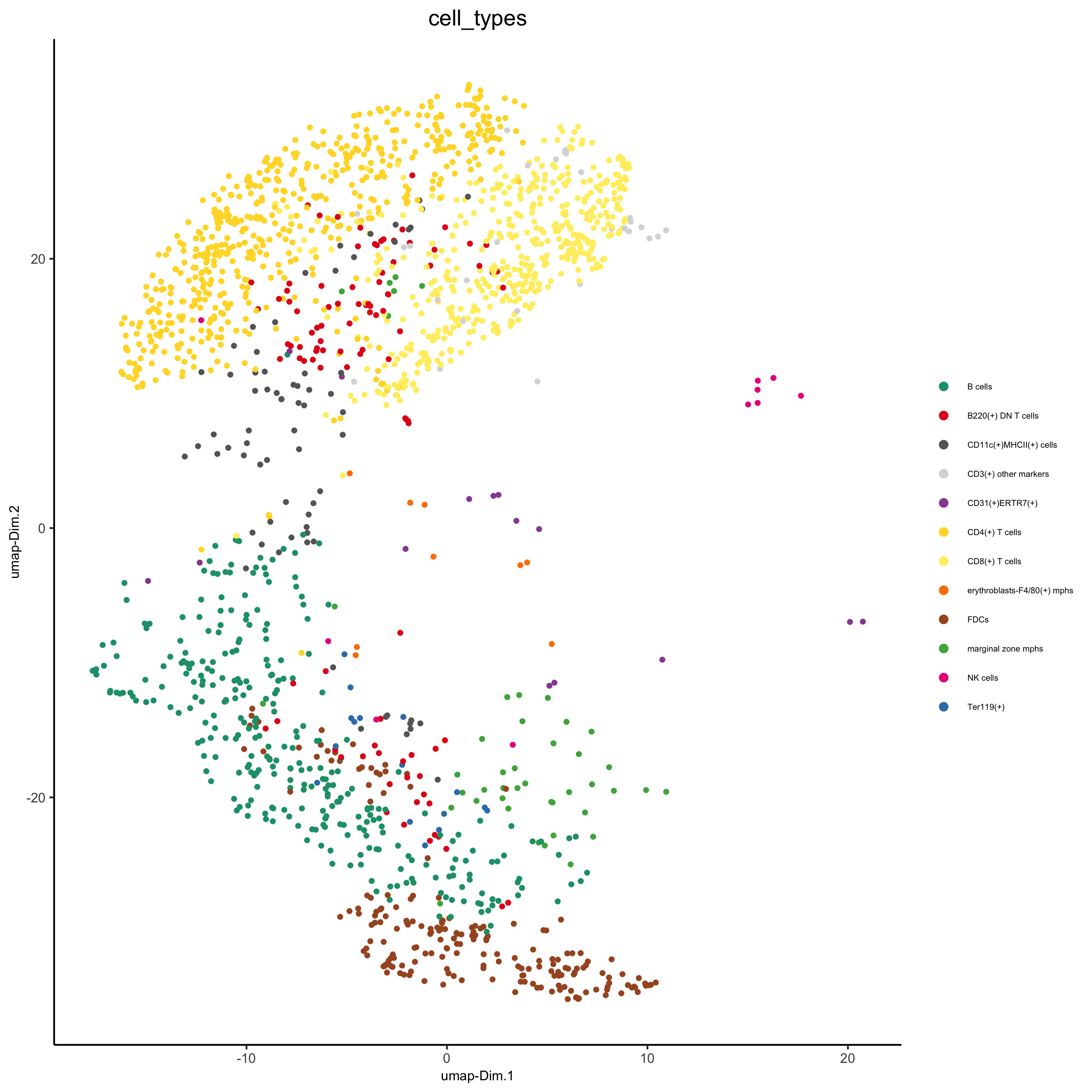
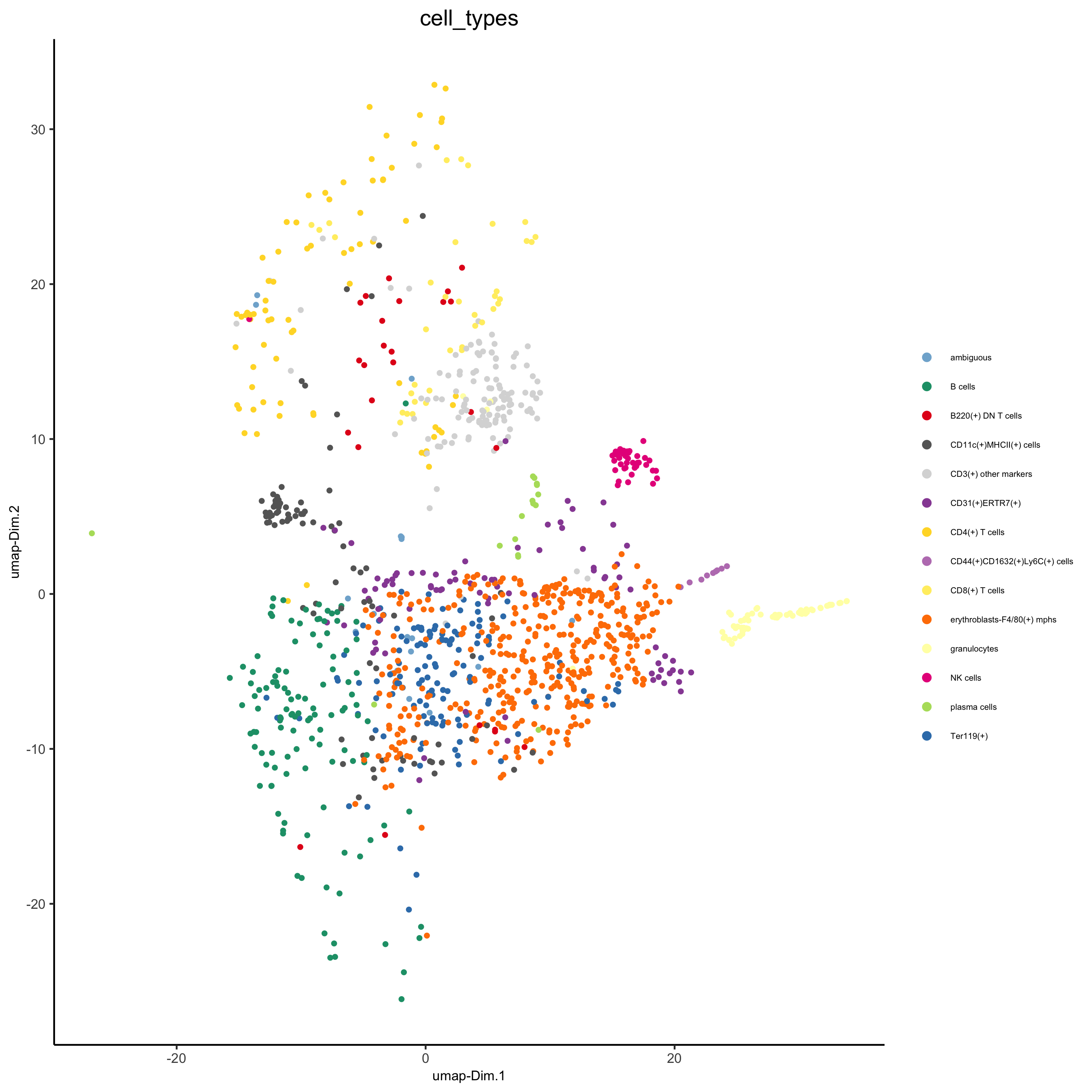
cell_metadata = pDataDT(codex_test) subset_cell_ids = cell_metadata[sample_Xtile_Ytile=="BALBc-3_X04_Y08"]$cell_ID codex_test_zone1 = subsetGiotto(codex_test, cell_ids = subset_cell_ids) plotUMAP(gobject = codex_test_zone1, cell_color = 'cell_types', point_shape = 'no_border', point_size = 1, cell_color_code = mycolorcode, show_center_label = F, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '8_a_umap'))
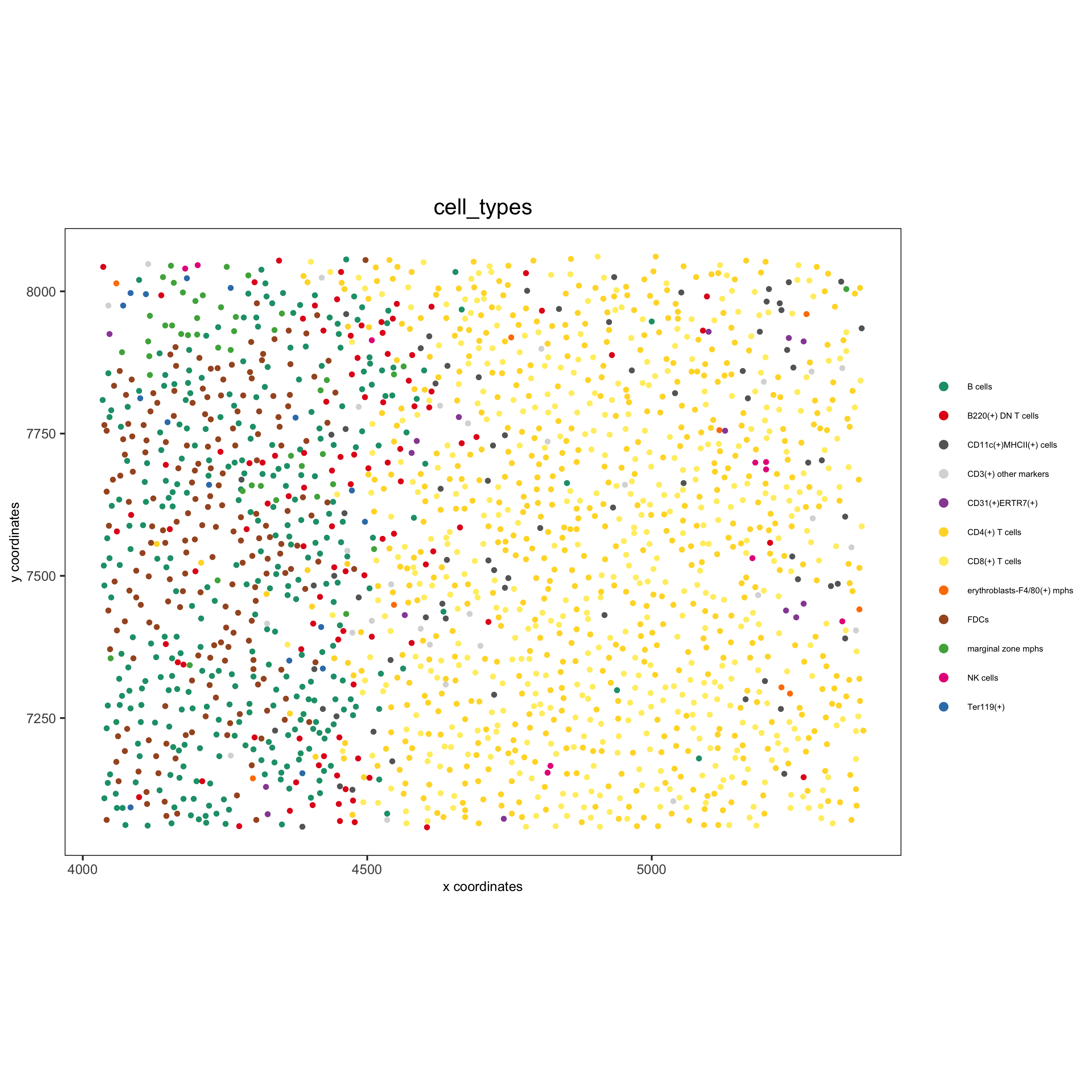
spatPlot(gobject = codex_test_zone1, cell_color = 'cell_types', point_shape = 'no_border', point_size = 1, cell_color_code = mycolorcode, coord_fix_ratio = 1, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '8_b_spatplot'))
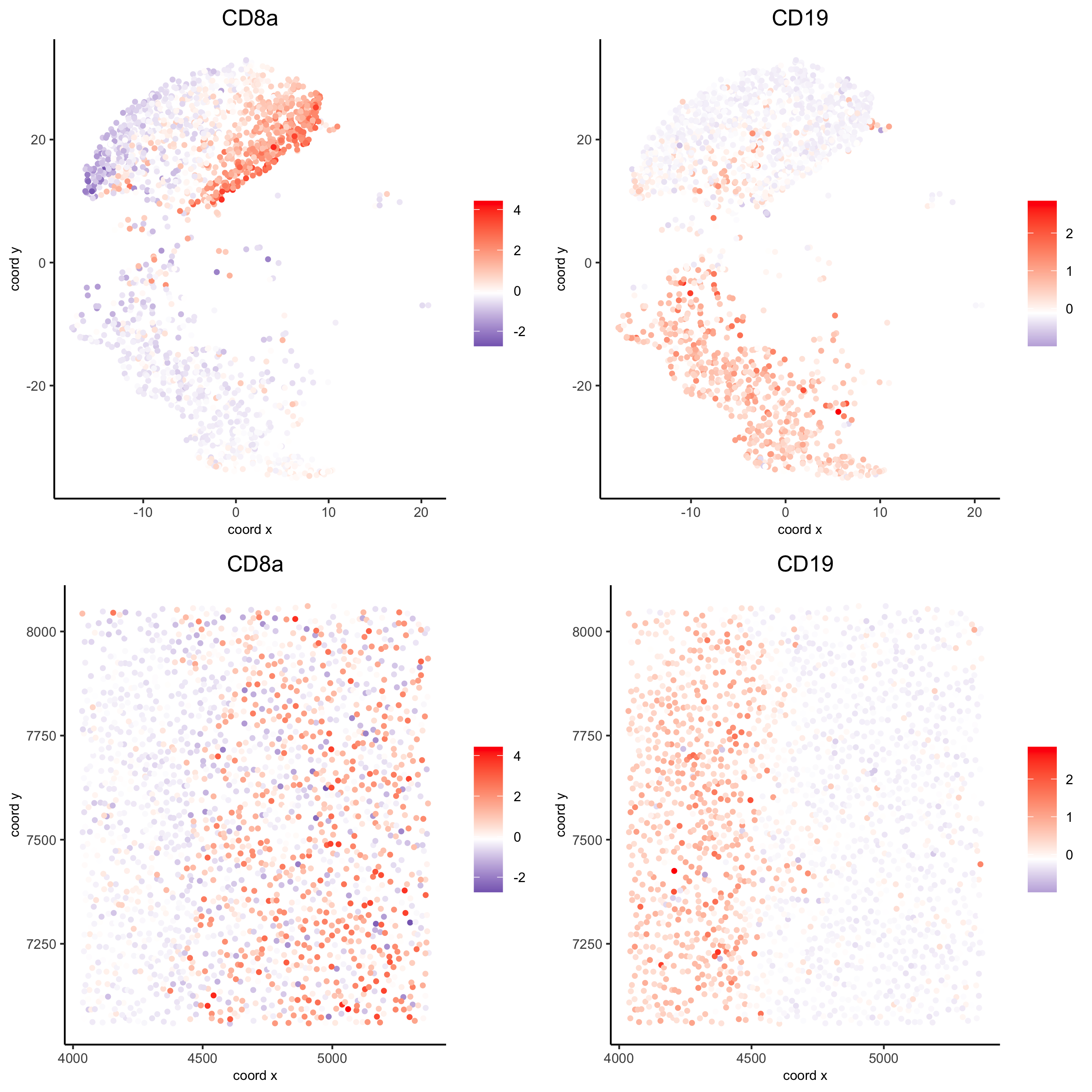
spatDimGenePlot(codex_test_zone1, expression_values = 'scaled', genes = c("CD8a","CD19"), spat_point_shape = 'no_border', dim_point_shape = 'no_border', cell_color_gradient = c("darkblue", "white", "red"), save_param = list(save_name = '8_c_spatdimplot'))
cell_metadata = pDataDT(codex_test) subset_cell_ids = cell_metadata[sample_Xtile_Ytile=="BALBc-3_X04_Y03"]$cell_ID codex_test_zone2 = subsetGiotto(codex_test, cell_ids = subset_cell_ids) plotUMAP(gobject = codex_test_zone2, cell_color = 'cell_types',point_shape = 'no_border', point_size = 1, cell_color_code = mycolorcode, show_center_label = F, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '8_d_umap'))
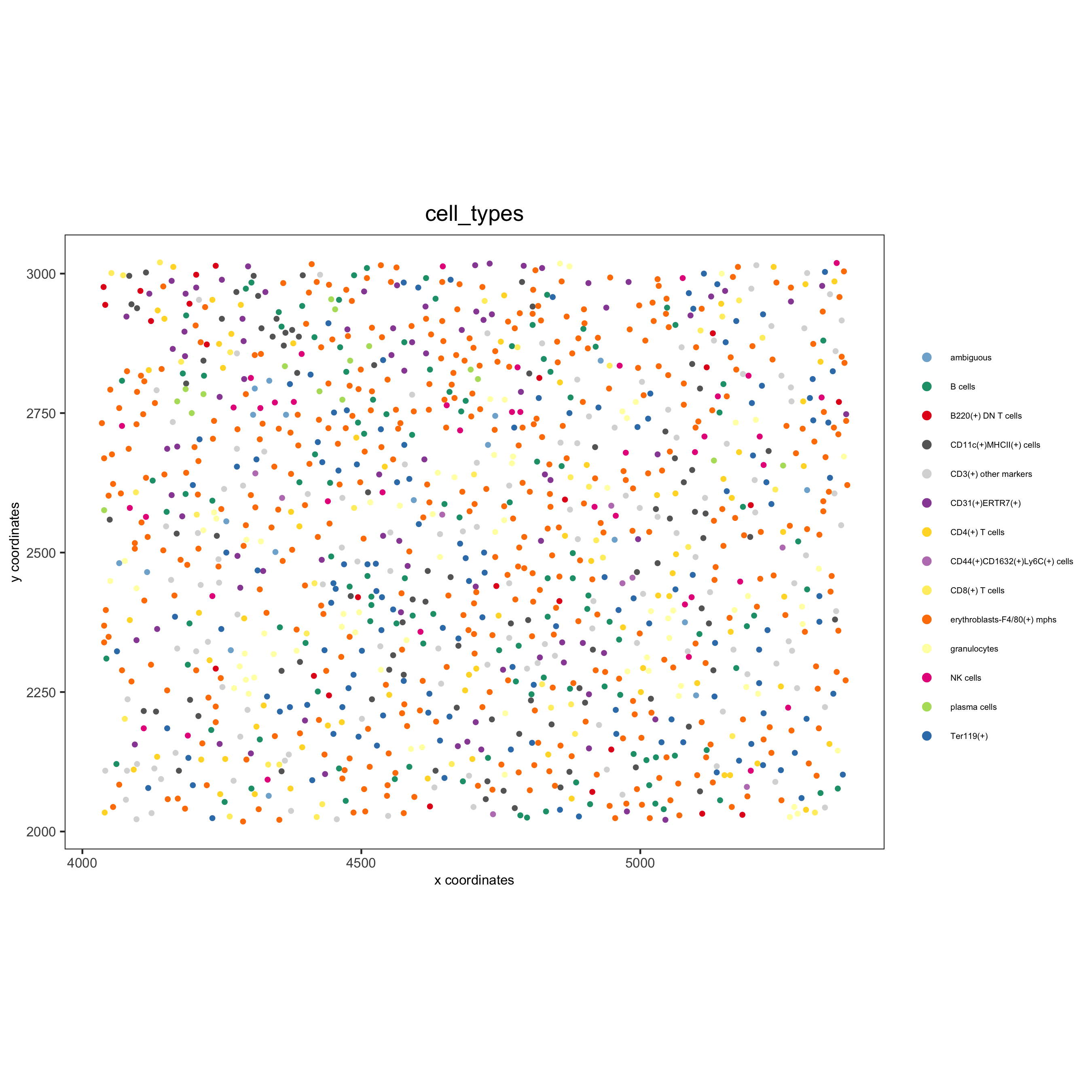
spatPlot(gobject = codex_test_zone2, cell_color = 'cell_types', point_shape = 'no_border', point_size = 1, cell_color_code = mycolorcode, coord_fix_ratio = 1, label_size =2, legend_text = 5, legend_symbol_size = 2, save_param = list(save_name = '8_e_spatPlot'))
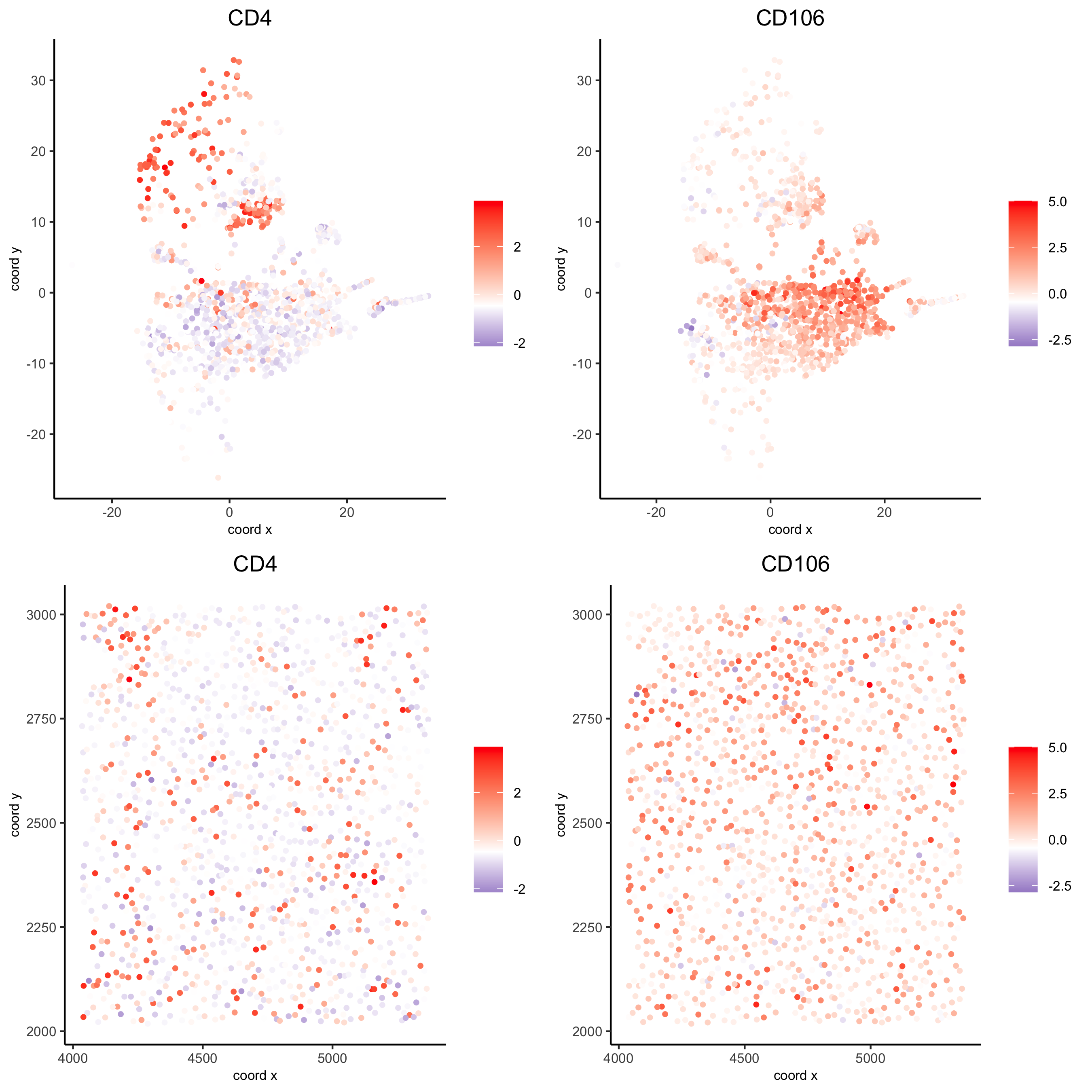
spatDimGenePlot(codex_test_zone2, expression_values = 'scaled', genes = c("CD4", "CD106"), spat_point_shape = 'no_border', dim_point_shape = 'no_border', cell_color_gradient = c("darkblue", "white", "red"), save_param = list(save_name = '8_f_spatdimgeneplot'))